Understanding Chemical Behavior
Summary:
The collection of experiments and data presented illustrates various fundamental principles in chemistry, such as the laws of conservation of mass, constant composition, multiple proportions, and reciprocal proportions. These laws govern the behaviour of elements and compounds during chemical reactions.
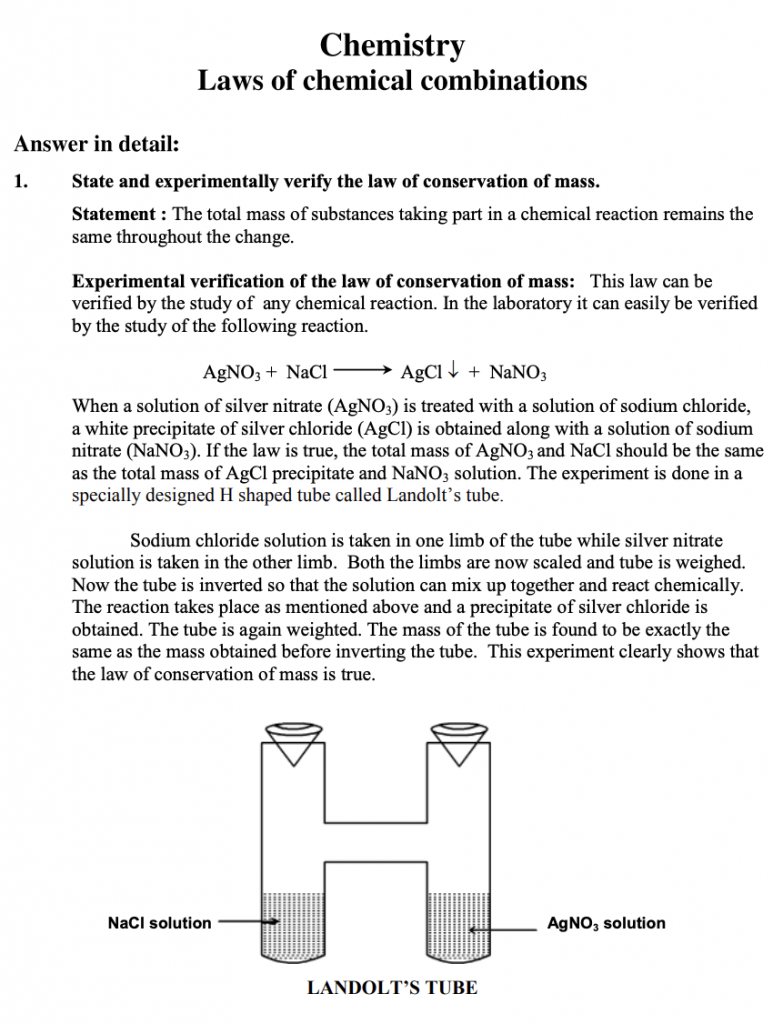
The law of conservation of mass is experimentally verified through observations of chemical reactions. For example, when silver nitrate and sodium chloride are combined, the total mass of the reactants equals the total mass of the products. This experiment demonstrates that the mass remains constant throughout the reaction.
The law of constant composition, also known as the law of definite proportions, states that compounds always contain elements in fixed ratios by weight. Experimental verifications of this law involve the analysis of compounds like cupric oxide and cupric nitrate. The ratios of copper to oxygen in these compounds are found to be constant, supporting the law of constant composition.
The law of multiple proportions is demonstrated by examining different oxides and their compositions. The weights of oxygen combined with fixed weights of nitrogen result in simple numerical ratios, indicating the law of multiple proportions.
In the law of reciprocal proportions, the ratios of elements combining separately with a third element are compared. Examples involving hydrogen, sulphur, and oxygen show that the ratios between hydrogen and sulphur, and sulphur and oxygen, are either the same or simple whole number multiples of each other.
The data presented in these experiments consistently support the laws of conservation of mass, constant composition, multiple proportions, and reciprocal proportions. These laws provide a fundamental understanding of chemical reactions and the behaviour of substances, forming the basis of modern chemistry.
Excerpt:
Understanding Chemical Behavior
1. State and experimentally verify the law of conservation of mass.
Statement: The total mass of substances taking part in a chemical reaction remains the same throughout the change.
Experimental verification of the law of conservation of mass: This law can be verified by studying any chemical reaction. In the laboratory it can easily be verified by the study of the following reaction.
AgNO3 + NaCl AgCl + NaNO3

Reviews