Solution Concentration and Stoichiometry
Summary:
This Solution Concentration and Stoichiometry note provide information on solution concentration and stoichiometry, specifically focusing on Molarity as a method of measuring concentration. It includes examples of how to calculate the molar concentration of a solution, as well as how to dilute a stock solution to a desired concentration. The material also discusses how to determine the molar concentration of each ion in a solution and how to use stoichiometry in reactions that occur in aqueous solutions.
Excerpt:
Solution Concentration and Stoichiometry
Molar Concentration of Solutions
The concentration of a solution is defined as the amount of solute dissolved in a given quantity of solvent or solution. Solution concentration can be defined in many ways (Molarity, normality, molality, per cent volume and per cent mass). This material focuses on Molarity.
Molarity (M) is the ratio of the number of moles of solute to the volume of the solution in litres.
Mathematically,
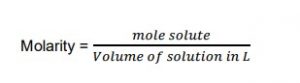
Solution Concentration and Stoichiometry
The unit for Molarity is mole/L.
To determine the molar concentration of a solution, its Molarity, we have to determine the number of moles of solute in the solution as well as the total volume of the solution in litres. We then simply determine the ratio of the number of moles of solute to the volume of the solution.
The molar concentration for solutions with a strong electrolyte solute can also be expressed in terms of the concentration of each ion. For example, if the concentration of a Sulfuric acid (H2SO4) solution is 0.32M, we can say that it is 0.32M in H2SO4, 0.64M in H+ (because there are 2 moles of hydrogen ions per mole of Sulfuric acid) and 0.32M in SO42-.
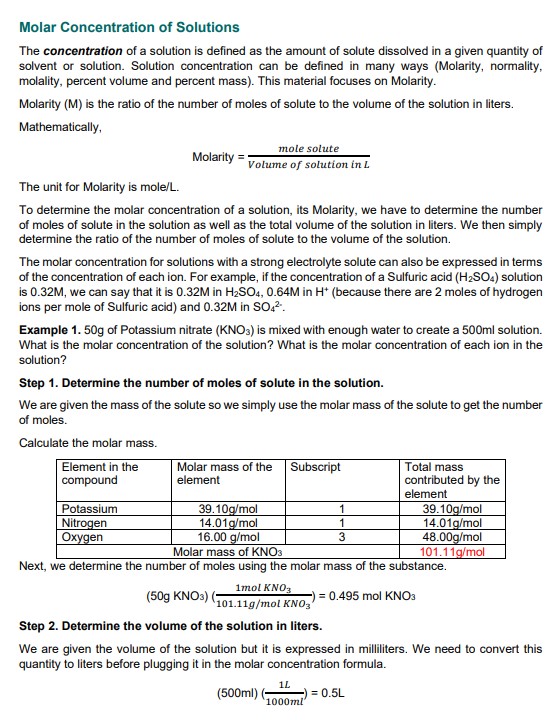
Example 1. 50g of Potassium nitrate (KNO3) is mixed with enough water to create a 500 ml solution. What is the molar concentration of the solution? What is the molar concentration of each ion in the solution?
Step 1. Determine the number of moles of solute in the solution.
We are given the mass of the solute, so we simply use the molar mass of the solute to get the number of moles.

Reviews