Percent Composition in Chemistry
Summary:
The Percent Composition in Chemistry discusses percent composition, which is the ratio of the mass of an element in a compound. The article provides examples of how to calculate percent composition using the formula and how to calculate the molar mass of a compound. It also shows how to organize calculations using a table. The article provides examples of calculating the percent composition of different compounds, including Ammonia, Sulfuric Acid, and Ammonium Sulfite. Finally, it provides the percentages of each element in these compounds by mass.
Additionally, understanding percent composition is essential for determining the stoichiometry of a chemical reaction, which involves balancing the number of atoms and molecules involved in a reaction. Percent composition also plays a crucial role in determining the physical and chemical properties of a compound, such as its melting point, boiling point, and reactivity. By understanding percent composition, chemists can predict the behavior of a compound in different conditions and develop new materials with specific properties. Therefore, mastering percent composition is an important step towards becoming a proficient chemist.
Excerpt:
Percent Composition in Chemistry
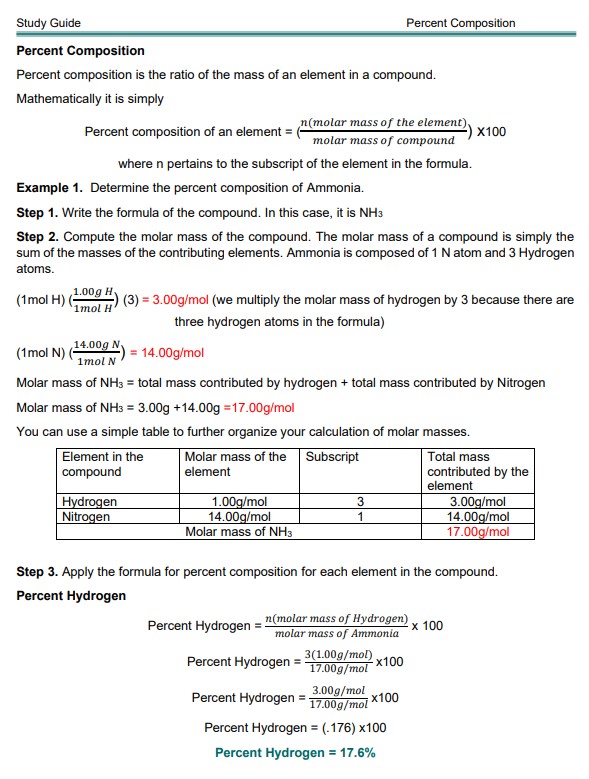
Percent composition is the ratio of the mass of an element in a compound. Mathematically it is simply
Percent composition of an element:
Percent Composition in Chemistry
Where n pertains to the subscript of the element in the formula.
Example 1. Determine the percent composition of Ammonia.
Step 1. Write the formula of the compound. In this case, it is NH3
Step 2. Compute the molar mass of the compound. The molar mass of a compound is simply the sum of the masses of the contributing elements. Ammonia is composed of 1 N atom and 3 Hydrogen atoms.

Percent Composition in Chemistry
You can use a simple table to organize your calculation of molar masses further.
| Element in the compound | The molar mass of the element | Subscript | Total mass contributed by the element |
| Hydrogen | 1.00g/mol | 3 | 3.00g/mol |
| Nitrogen | 14.00g/mol | 1 | 14.00g/mol |
| The molar mass of NH3 | 17.00g/mol | ||

Reviews