Introduction to Polymer Chemistry (Grade A)
Summary:
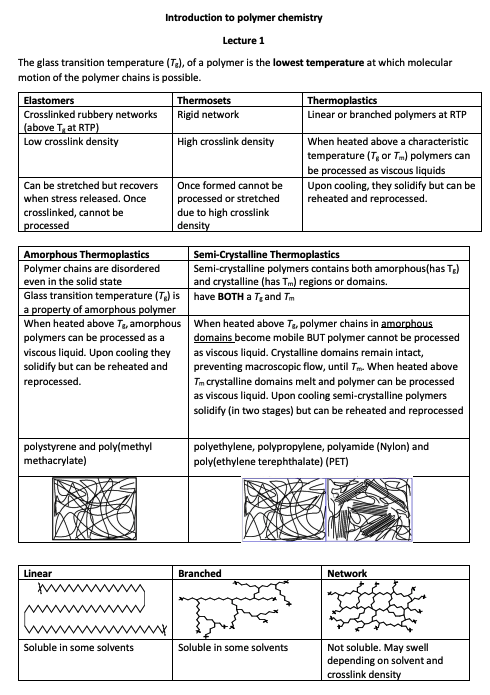
Polymers are large macromolecules composed of repeating units called monomers. A crucial property of polymers is the glass transition temperature (Tg), the minimum temperature at which polymer chains can move. Polymers are elastomers, thermosets, and thermoplastics, each having unique physical properties and behaviours. For example, thermoplastics can be heated and reshaped multiple times, whereas thermosets, once formed, cannot. The structure and nature of polymers, including chain density and branching, influence their characteristics, like melting temperature. A notable distinction exists between amorphous polymers, which are disordered even in solid states, and semi-crystalline polymers, which contain disordered (amorphous) and ordered (crystalline) regions. Polymers’ molar mass and distribution play a vital role in their physical properties and can be assessed using techniques like size exclusion chromatography (SEC). Other crucial properties include tensile strength, quantifying a material’s resistance to breakage under tension. In-depth thermal analyses, like Dynamic Scanning Calorimetry and Dynamic Mechanical Analysis, provide insights into the polymers’ behaviour at various temperatures, emphasizing their Tg or melting points.
Excerpt:
Introduction to Polymer Chemistry
….
Cellulose
Cellulose is a straight-chain polymer: unlike starch, no coiling occurs, and the molecule adopts an extended rod-like conformation. This optimises hydrogen bonding and makes cellulose completely insoluble in water. Hydrogen bonding is both Intramolecular and Intermolecular. This gives cellulose great tensile strength – a structural polymer in plant cell walls. The most common organic compound on earth and cellulose makes up. A third of all plant matter is 90% of cotton fibre, 50% of wood (with lignin)
Starch
Starch consists of two types of molecules: Amylose and Amylopectin. Both consist of polymers of α-D-glucose. In amylose, all glucose units are linked -(1,4)- with the ring oxygen atoms on the same side. In amylopectin, about one glucose residue in every twenty or so is also linked -(1,6)- forming branch points.
The relative proportions of amylose to amylopectin depends on the source of the starch; for example, Amylomaizes contain over 50% amylose. Whereas ‘waxy’ maize has almost none (~3%).

Reviews