IB HL Chemistry Key Concepts
Summary:
The IB HL Chemistry Key Concepts provided text encompasses various chemistry-related topics, including polyatomic ions, resonance, ozone depletion, acidic and basic oxides, functional groups, oxidation states, rate graphs, nucleophilic substitution, and nitrogenation of benzene. While it’s challenging to condense the content into a comprehensive 300-word summary, I will attempt to provide a general overview.
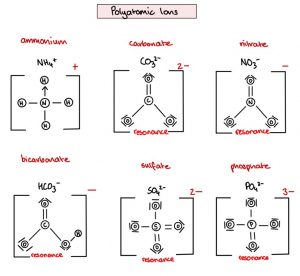
The text begins by mentioning polyatomic ions and their resonance structures. It then touches upon the concept of ozone depletion and the role of catalysts like Cl2 and NO2 in the process.
Next, it delves into classifying oxides as basic or acidic and provides examples such as sodium oxide (Na2O) as a basic oxide and carbon dioxide (CO2) as an acidic oxide. The summary further highlights the reactions of these oxides with water to form hydroxides or acids, such as sodium hydroxide (NaOH) from Na2O and magnesium hydroxide (Mg(OH)2) from MgO.
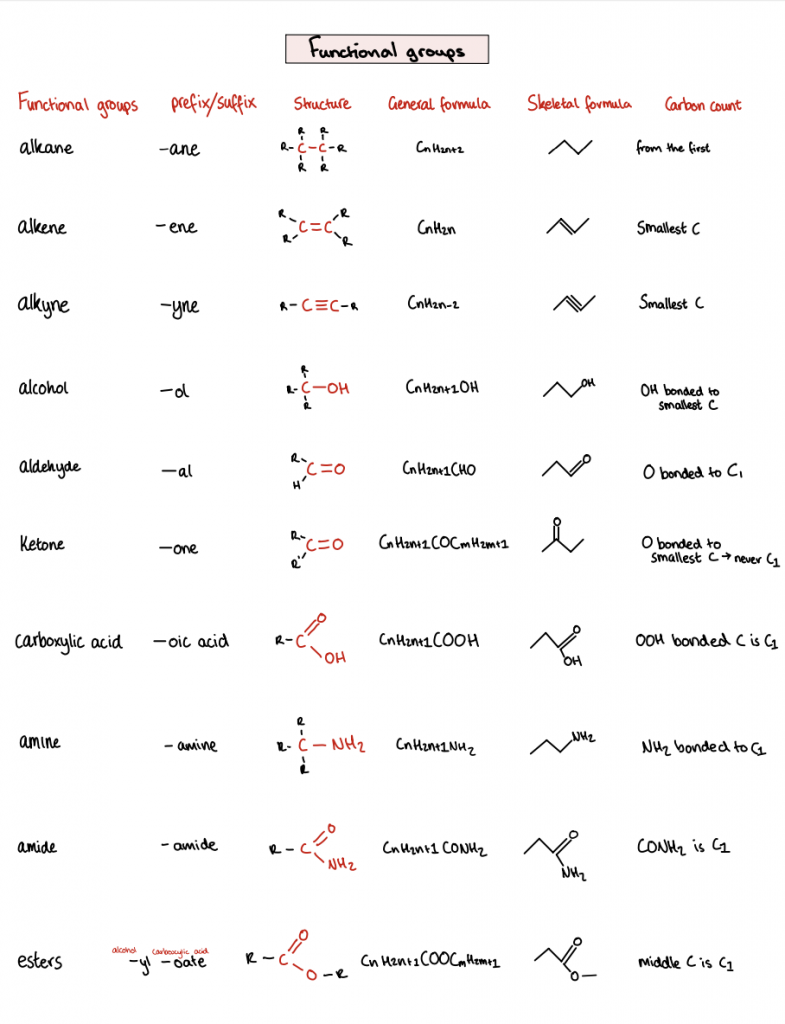
Functional groups are briefly mentioned, including alcohols, aldehydes, ketones, carboxylic acids, amines, and amides. Specific suffixes and prefixes, with examples for each, characterize these groups.
The text then introduces the concept of oxidation and the different oxidation states of elements. It covers the oxidation of alcohols to aldehydes or ketones and, further, to carboxylic acids. Additionally, it mentions the oxidation of sulphurous acid (H2SO3) to sulfuric acid (H2SO4).
Rate graphs and reaction orders are touched upon, including zero-order, first-order, and second-order reactions. These graphs illustrate the relationship between reactant concentration and time in different types of reactions.
Nucleophilic substitution, both unimolecular (SN1) and bimolecular (SN2), is discussed briefly, emphasizing the role of nucleophiles as electron-rich species with lone pairs. The influence of solvent polarity on the favouring of SN1 or SN2 reactions is mentioned.
Lastly, the text briefly covers the nitrogenation of benzene, involving the formation of the nitronium ion (NO2+) and subsequent electrophilic substitution reactions to produce nitrobenzene. The reduction of nitrobenzene to phenylamine is also mentioned.
Excerpt:
IB HL Chemistry Key Concepts
IB HL Chemistry Key Concepts

Reviews