IB Chemistry Periodicity (Periodic Table)
Summary:
The IB Chemistry Periodicity note covers various topics related to the periodic table and its elements. It explains the arrangement of elements in the periodic table based on atomic number and distinguishes between groups and periods. It also covers physical properties such as first ionization energy, electronegativity, and trends in atomic and ionic radii across groups and periods. The blog also discusses the chemical properties of elements in the same groups, such as the reactions of alkali metals with water and halogens, and the changes in the nature of oxides across period 3.
Excerpt:
IB Chemistry Periodicity (Periodic Table)
3.1 – The Periodic Table
3.1.1 – Describe the arrangement of elements in the periodic table in order of increasing atomic number
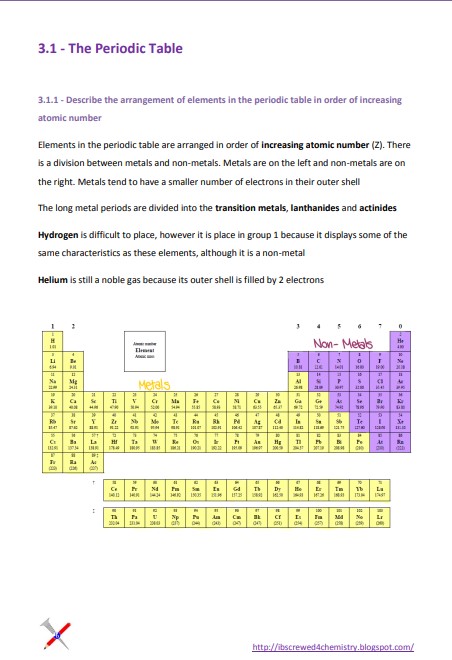
Elements in the periodic table are arranged in order of increasing atomic number (Z). There is a division between metals and non-metals. Metals are on the left and non-metals are on the right. Metals tend to have a smaller number of electrons in their outer shell The long metal periods are divided into transition metals, lanthanides, and actinides
Hydrogen is difficult to place, however, it is placed in group 1 because it displays some of the same characteristics as these elements, although it is a non-metal. Helium is still a noble gas because its outer shell is filled with 2 electrons.
IB Chemistry Periodicity (Periodic Table)
3.1.2 – Distinguish between the terms group and period
Group – A vertical column of elements.
These have been classified in a number of ways – IB numbers them 1 to 7, with the noble gases being group 0. Some groups have names, such as Alkali metals (group 1) and Halogens (group 7). Groups 3 to 6 have both metal and non-metal elements. The metalloids (B, Si, Ge, As, Sb, Te, and Po) have characteristics of both metals and non-metals.
Period – A horizontal row of elements
These are numbered 1 to 7. The number matches the number of its outer shell electrons. Elements of the same period have the same number of occupied electron shells.

Reviews