BCH 2333 Intro to Biochemistry (Grade A)
Summary:
In this laboratory experiment, the objective was to separate a mixture of glycine, aspartic acid, and lysine using ion exchange chromatography. The pH and elution volume of the fractions obtained during the separation were measured. The experiment also involved performing a Ninhydrin test to determine the concentration of the amino acids. In addition, sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was used to determine the molecular weight of an unknown protein. By analyzing the migration distance of the protein sample and using a linear regression model, the molecular weight of the unknown protein was calculated. The experiment aimed to illustrate the elution behaviour of amino acids based on their isoelectric points and to determine the molecular weight of the unknown protein.
Excerpt:
BCH 2333 Intro to Biochemistry
Laboratory BCH2733
LAB 2: Amino Acids and Proteins
Purpose
The first objective was to separate a glycine, aspartic acid and lysine mixture using an ion-exchange
chromatography. The pH of the sixteen fractions resulting from the separation was obtained. These pH
values along with the elution volume helped illustrate how through a gradient of increasing pH, the amino
acid was eluted based on their isoelectric point. A Ninhydrin test followed by the heating of the amino
acid samples was also performed to assess the amino acid concentration by spectrophotometry. The
second objective was to find the molecular weight of an unknown protein using sodium dodecyl sulphate
polyacrylamide gel electrophoresis (SDS-PAGE). As the unknown protein separated by electrophoresis,
the molecular weight markers traced the migration of the protein along the gel. The end result of that
made it possible to model the linear relationship between the logarithm of the protein molecular weight
and its migration distance. By interpolation, the molecular weight of the unknown protein was then found.
For more information, please refer to “Introduction to Biochemistry – Laboratory Component”, pg. 29-43.
BCH 2333 Intro to Biochemistry
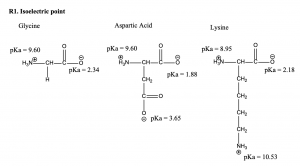
Glycine: pH = (0.5) (9.60+2.34) = 5.97
Aspartic acid: pH = (0.5) (3.65 +1.88) = 2.77
Lysine: pH = (0.5) (8.95+10.53) = 9.74
The isoelectric point for glycine, aspartic acid and lysine are 5.97, 2.77, and 9.74 respectively.
(The pKa values are obtained from the class notes)


Reviews