Aqueous Solutions and Precipitation Reactions
Summary:
The Aqueous Solutions and Precipitation Reactions study guide discusses solutions, electrolytic and non-electrolytic solutions, precipitation reactions, and solubility. It also provides a table of strong electrolytes, weak electrolytes, and non-electrolytes. The solubility guide lists soluble and insoluble ionic compounds and their exceptions. The guide provides examples of predicting whether or not a precipitate will form in a reaction and how to write the net ionic equation.
Excerpt:
Aqueous Solutions and Precipitation Reactions
Solutions are homogeneous mixtures of two or more substances. The substance present in a smaller amount in the mixture is called the solute, while the substance that has a greater amount is the solvent. The solute is dissolved in the solvent. Homogenous means that you cannot identify the particles of the solute and the solvent separately in the solution. Mixture means that the solute and the solvent retain their individual properties and that the solute and the solvent are only physically combined. A solution can be gaseous like air, liquid like soda or solid like metal brass (an alloy of copper and zinc).
Aqueous solutions are solutions in which the solvent is water.
Electrolytic solutions are solutions that can conduct electricity due to the presence of dissolved ions.
Non-electrolytic solutions are solutions that cannot conduct electricity because the solutes do not have ions when dissolved in water.
The electrolyte is a solute that results in an electrolytic solution when dissolved in water.
Non-electrolyte is a solute that does not result in an electrolytic solution when dissolved in water
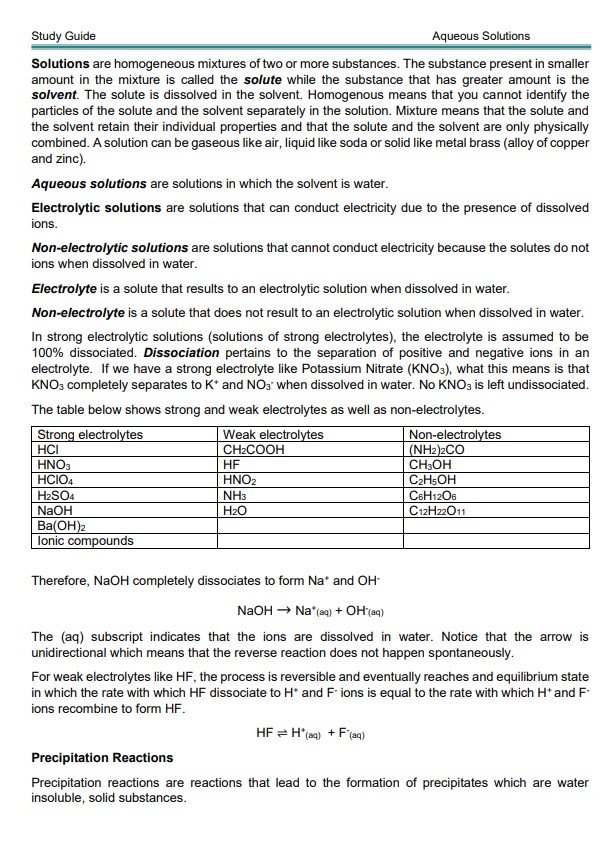
In strong electrolytic solutions (solutions of strong electrolytes), the electrolyte is assumed to be 100% dissociated. Dissociation pertains to the separation of positive and negative ions in an electrolyte. If we have a strong electrolyte like Potassium Nitrate (KNO3), this means that KNO3 completely separates into K+ and NO3 – when dissolved in water. No KNO3 is left undissociated.

Reviews