Acid Base and Salt in Class 10 Chemistry
Summary:
This is a study material on “Acid, Bases, and Salts” for Class 10 students. It provides information about acids, bases, alkalis, salts, and their chemical properties. The content covers reactions involving acids and bases, the concept of pH, indicators, strong and weak acids/bases, and the importance of pH in everyday life. The summary also includes information about some important salts, such as common salt, bleaching powder, baking soda, washing soda, and plaster of Paris. It touches on their chemical nature and common uses. Additionally, the concept of water crystallization in hydrates is mentioned. The material appears to be aimed at providing comprehensive information for students studying this topic in their 10th-grade class.
Excerpt:
Acid Base and Salt in Class 10 Chemistry
What you will get
❖Premium Lectures
❖Class notes
❖PYQs (chapters)
❖ Competency-based questions
What is an Acid
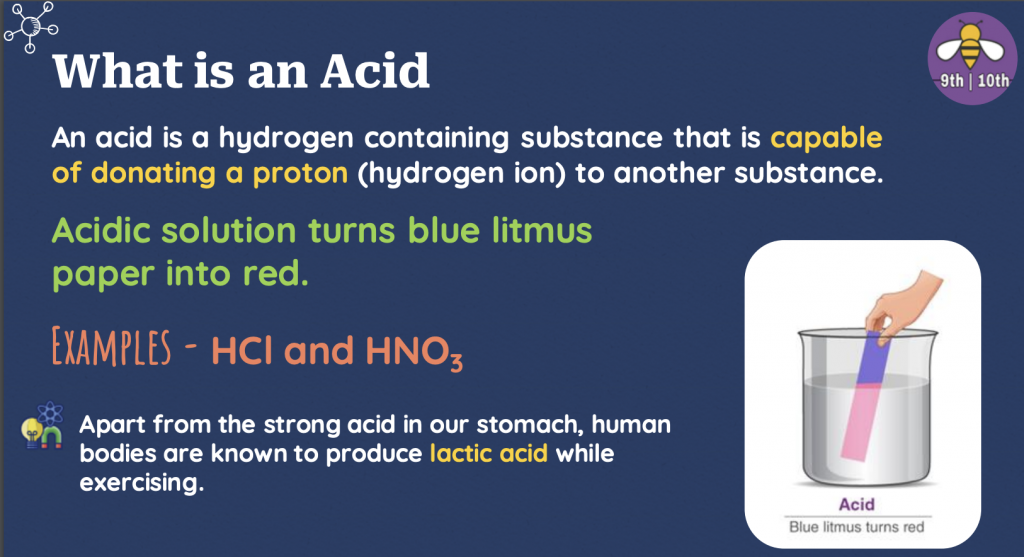
– An acid is a hydrogen-containing substance capable of donating a proton (hydrogen ion) to another substance.
– The acidic solution turns blue litmus paper into red.
Examples – HCl and HNO3
Besides the strong acid in our stomach, human bodies produce lactic acid while exercising.
What is a Base
Bases are chemical substances that taste bitter, are soapy to touch and turn red litmus blue. It
dissolves in water to produce hydroxide ions (OH-) in solutions.
Basic solution turns red litmus paper into blue.
Examples – NaOH → Na(aq) + OH-
What are Alkalis
Bases that are soluble in water are called Alkalis.
Examples – Sodium hydroxide
(NaOH), Potassium hydroxide
(KOH), Calcium hydroxide
[Ca(OH)2]
What are Salts
Salts are produced due to the reaction between acids and bases.
Chemical properties of acids and bases
Acid + Bases → Salt + Water
Acid + Metal
Acid + Metal Oxide
Acid + Metal Carbonate/Metal bicarbonate
Chemical Properties of Acids and Bases
Acid + Bases → Salt + Water
Base + Metal
Base + Non – Metal Oxide
The Reaction of Acid and Base with Metals
Zn(s) + H2SO4
(aq) → ZnSO4
(aq)+ H2 (g)
EXAMPLES
Acid + Metal → Salt + Hydrogen gas
NaOH(aq) + Zn(s) → Na2ZnO2 (aq)+ H2 (g)

Reviews